The last post was about my class in medical school and the president of the class, David Mrazek, who was struck down by cancer at the height of his contribution to the medical community. We loved David and worked with him to support each other. The link below from one of his colleagues is a testimony to his continued contributions. David was a leader in understanding the root causes of diseases of the brain. Unfortunately, it was one of those diseases that killed him. “After receiving the diagnosis of glioblastoma in his 64th year of life, David struggled courageously with his new reality. He and his family used all means, conventional and experimental, to fight his cancer in hopes of extending his life. Unfortunately, this was not to be.” David handled the end with as much class as he handled everything else.
By now, those of you who read this content know that I am primarily focused on cardiovascular and related diseases like diabetes, but studying these conditions has taught me that all chronic diseases and aging are related. I have yet to find an exception. Like David, I have become increasingly interested in the root causes of chronic diseases including genetics, epigenetics, molecular biology, and environmental factors. I had not yet studied glioblastoma, so I decided to examine the underlying mechanisms of that disease. It has taken other people you know like Ted Kennedy, Beau Biden, John McClain, and Tim Conway. It is an awful disease. It always recurs, and average survival is about 15 months.
The human genome project was completed in 2003, about ten years before David’s death. We thought that mapping out the entire human genome would give us the answers to the causes and cures of chronic disease like cancer. It made sense, but it did not pan out. It turns out that cancer is not mostly about changes in the DNA itself. It is not related to gene mutations as much as it is with gene regulation. Glioblastoma is no exception.
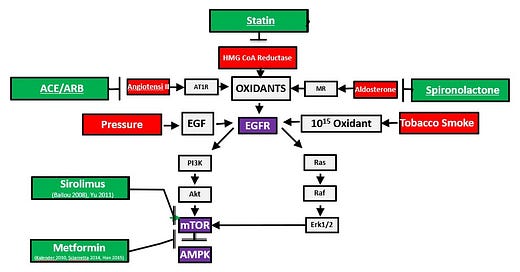
I developed the slide above the explain the core epigenetic and signaling pathways involved in cardiovascular disease and related conditions like diabetes. As you can see in the upper left corner of the diagram, angiotensin II engages the AT1 receptor on the cell surface and that leads to increased oxidant production. Higher oxidant levels activate the EGFR (epidermal growth factor) receptor and other growth factors. These in turn, switch on the master metabolic genetic switch mTOR (the mechanistic target of rapamycin). When mTOR is switched on persistently, that supports wild growth in the tumor. This is one of the key mechanisms in cardiovascular disease, diabetes, hypertension, and glioblastoma.
It is important to realize the early problem is not abnormal genes or genetic mutations. It is abnormal gene regulation. The genes that produce angiotensin II are normal genes. They are critical to fetal and childhood development. If the fetus cannot make angiotensin II or you block it with a medication like lisinopril or losartan, there will be a birth defect involving the kidney and draining system. This system and the other factors we will discuss exist to form a normal healthy child. They become less active in young healthy adults only to be reactivated later in life by belly fat, smoking cigarettes, and aging. Blocking the effect of angiotensin II later in life slows progressive age-related organ damage in the heart kidney and brain. Aging is the consequence of accumulation of damaged DNA, proteins, and fats, which are modified by excess oxidant production. Oxidants are the most prominent molecules involved in the aging process.
Angiotensin II leads to oxidant production, mitochondrial damage, and more oxidant production in a vicious cycle. Oxidants cause DNA strand breaks and multiple genetic mutations. Changes in gene regulation and genetic mutations accumulate and form cancers like glioblastoma. Losartan blocks the effects of angiotensin II precisely and there are reports of losartan preventing glioblastoma or being used in combination with other treatments. Lisinopril, statins, metformin, spironolactone, and eplerenone are all powerful antioxidants that protect against the inflammation and wild cell growth that are part of the malignant process. There is more evidence that these antioxidants and lifestyle measures have an important impact. When these medications are combined in optimal medical therapy in patients who have had a heart attack, there is a ten-fold difference in cardiovascular and all-cause mortality over the next five years.
Don’t get bogged down in the nerdy science here. My purpose is to help you understand that glioblastoma, like other chronic diseases, is related to core genetic, epigenetic, and signaling pathways Metformin is an especially interesting story in this disease. We think of metformin as a diabetes drug that lowers insulin resistance in the liver. It does that, but it does so much more. Metformin directly blocks the effects of asymmetric dimethyarginine (ADMA) which is an amino acid derived from arginine. The enzyme that makes most ADMA (PRMT1) is essential to fetal development. A fetus without that enzyme only survives a matter of days. This enzyme is inappropriately switched on in glioblastoma. Metformin blocks the effects of ADMA to directly switch off mTOR (master genetic fetal growth switch) and switch on AMPK (master genetic fetal survival switch). Daily aspirin, which also switches off mTOR and switches on AMPK reduces the risk of glioblastoma by about a third. Other key molecules like NF‑κB (inflammation), Wnt, PI3K/AKT/mTOR (lower left of the above diagram and supports wild growth) are inappropriately switched on in glioblastoma.
There are many genes inappropriately switched on or mutated in glioblastoma. That is why treatments aimed at one factor are unsuccessful. The increased oxidant production in the slide above activates growth factor signaling through the epidermal growth factor receptor (EGFR). Transforming growth factor B (TGF-B) and vascular epidermal growth factor (VEGF) are also switched on in glioblastoma and can be substituted in the EGFR position in the diagram. As you can easily see, this deadly tumor is all about genetics, gene regulation and the excess oxidant production from these changes. That is how we must study chronic illnesses to make progress. When we get more people on appropriate optimal medical therapy (OMT) for cardiovascular and related diseases, we will develop the big data that shows the impact of this intervention in preventing dreaded diseases like glioblastoma. Let’s get started.




Hi Bill. This is a surprising connection. Have you investigated similar connections between optimal health and Musculoskeletal conditions? Keep up the good work! - Steve
Thanks Laura